CDC conducts studies each flu season to help determine how well flu vaccines are working. These vaccine effectiveness (VE) studies help regularly assess the value of flu vaccination as a public health intervention. The results of vaccine effectiveness studies can vary based on the study design, the outcome(s) measured, the population studied, and the season studied.
U.S. Flu Vaccine Effectiveness Networks
CDC has been working with researchers at universities and hospitals since the 2003-2004 flu season to estimate how well flu vaccines work through observational studies using laboratory-confirmed flu as the outcome. Over the past few years, CDC has conducted VE studies using multiple vaccine effectiveness networks. More information on CDC’s vaccine effectiveness networks and studies is available at CDC’s Influenza Vaccine Effectiveness Networks.
Results from Prior Flu Seasons
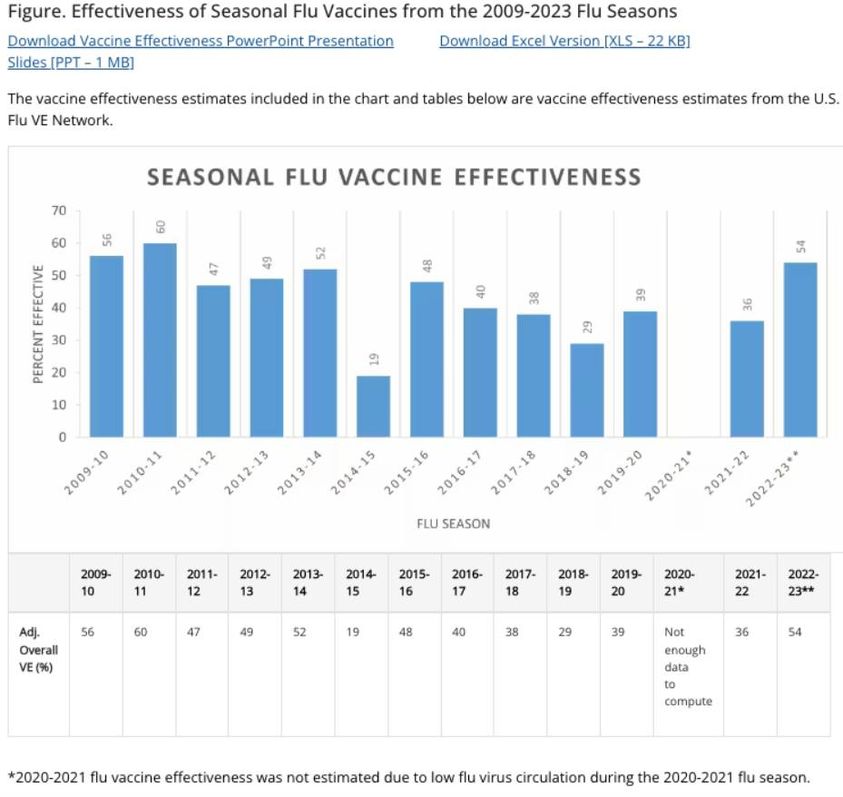
The overall, adjusted vaccine effectiveness estimates for flu seasons from 2004-2023 are noted in the chart below. (Estimates are typically adjusted for study site, age, sex, underlying medical conditions, and days from illness onset to enrollment.)
Figure. Effectiveness of Seasonal Flu Vaccines from the 2009-2023 Flu Seasons
Download Vaccine Effectiveness PowerPoint Presentation Slides [PPT – 1 MB]
Download Excel Version [XLS – 22 KB]
The vaccine effectiveness estimates included in the chart and tables below are vaccine effectiveness estimates from the U.S. Flu VE Network.
Source: https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm
